23 February, 2026
Developed supercomputer-based simulation techniques
Fri 09 Jan, 2026
Context:
- Indian scientists have, for the first time, developed supercomputer-based simulations to understand the long-standing paradox of the Mpemba Effect.
Key Points:
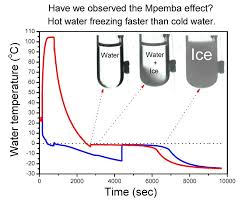
- Researchers from Bengaluru (an autonomous institution under the Department of Science and Technology – DST) have, with the help of a supercomputer, developed the first highly detailed simulation of ice formation in water that clearly demonstrates the Mpemba Effect.
- This effect becomes clearly visible under certain specific conditions when the process starts with hot water.
- The Mpemba Effect is not limited to water alone—it can also occur in other substances during the fluid-to-solid phase transition.
- The hydrogen bonds, metastable states, and the dynamics of nucleation (the initial formation of ice) in water play a crucial role in this phenomenon.
- The simulation accounts for the anomalous properties of water (such as maximum density at 4°C and its hydrogen-bond network), which distinguish it from other liquids.
Why is this Achievement Important? :
- It helps resolve a decades-old scientific controversy using computational methods.
- Such detailed simulations of a complex system like water were not previously possible due to the extremely high computational power required, which is now achievable through supercomputers.
- The study suggests that the Mpemba Effect may be a general non-equilibrium phenomenon, observable in various types of materials.
- In the future, this research may open new applications in cryogenics, food preservation, material science, and quantum systems.
Mpemba Effect:
- The Mpemba Effect refers to the phenomenon in which hot water freezes faster than cold water under certain conditions.
- Discovery: It is named after Erasto Mpemba, a student from Tanzania, who in 1963 observed during a cooking class that a hot milk mixture froze faster than a cold one.
- Historical Background: Although named after Mpemba, this phenomenon had been mentioned centuries earlier by scientists such as Aristotle, Francis Bacon, and René Descartes.
How Does This Effect Work? (Possible Scientific Explanations):
- Evaporation: Hot water evaporates more rapidly, reducing the total amount of water. A smaller volume can freeze faster.
- Convection: Hot water develops stronger convection currents, allowing heat to reach the surface and escape more quickly.
- Dissolved Gases: Cold water contains more dissolved gases, which can slow down the freezing process. Heating drives these gases out.
- Hydrogen Bonding: According to modern research, hydrogen bonds between water molecules expand when heated and then contract rapidly while releasing energy, thereby accelerating the freezing process.
Examples from Daily Life:
- Ice Cream Making: Many confectioners or chefs believe that placing a hot mixture in the freezer can help it freeze faster and better (though this is not ideal for the freezer).
- Ice Trails: In extremely cold regions, people sometimes throw boiling water into the air for entertainment, where it instantly turns into ice.