21 September, 2025
Discovery of a New Behavior in the Aggregation of Gold Nanoparticles (AuNPs) under the Influence of Amino Acids and Salts
Sun 14 Sep, 2025
Context:
- Scientists from S. N. Bose National Centre for Basic Sciences (DST Institute), Kolkata have discovered a new behavior in the aggregation of gold nanoparticles (AuNPs) influenced by amino acids and salts.
Key Points:
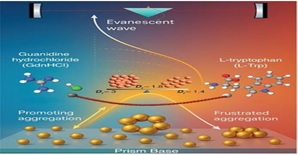
- Scientists found that everyday molecules — such as amino acid (L-Tryptophan) and a commonly used laboratory salt (Guanidine Hydrochloride / GdnHCl) — can control the clustering (aggregation) process of gold nanoparticles.
- When GdnHCl alone is added, nanoparticles form dense clusters quickly, changing their optical and color properties — which can be harmful for the stability of biosensors and optical tools.
- When both L-Tryptophan and GdnHCl are added together, instead of dense clusters, open, branch-like structures are formed. Scientists have named this process “frustrated aggregation” — meaning particles attempt to fully stick together, but the amino acid (L-Trp) obstructs the process.
Technical Aspect and Method:
- The team used one of the world’s most sensitive optical techniques — Evanescent Wave Cavity Ringdown Spectroscopy (EW-CRDS) — which revealed that L-Trp slows down the effect of GdnHCl and maintains the clusters as open and stable.
- This method can measure real-time updates in nanoparticle behavior.
Applications:
- Biosensors: Virus detection
- Diagnostics: Imaging
- Drug Delivery: Targeted therapy
- Environment: Pollutant detection
Gold Nanoparticles (AuNPs) :
- These are nanoscale particles made of gold (Au).
- Their size ranges from 1 to 100 nanometers (nm).
Structure:
- Gold atoms combine to form nanoparticles in shapes such as spheres, rods, shells, and stars.
- Their color is not the typical yellow of bulk gold but can appear red, purple, or blue.
Properties:
- Optical Properties (Surface Plasmon Resonance - SPR): Their color changes due to SPR.
- Chemical Stability: Gold is inert, making AuNPs stable.
- Surface Modification: Their surface can be easily functionalized with biomolecules (DNA, proteins) or drugs.
- Biocompatibility: Safe to use inside the human body.
Synthesis Methods:
- Chemical reduction (e.g., citrate reduction method – the most common).
- Biological synthesis (green synthesis using plant extracts, microorganisms).
- Physical methods (laser ablation, UV irradiation, etc.).
Applications:
Medical and Diagnostics:
- Identification and treatment of cancer cells (drug delivery, photothermal therapy).
- Biosensors (e.g., used in COVID-19 test kits).
- Imaging (MRI/CT scan contrast agents).
Industry and Technology:
- Electronics and nano-circuits.
- Chemical catalysis.
- Environmental pollution detection.
Research and Education:
- Used as model particles in nanotechnology research.
Unique Properties of Gold Nanoparticles (AuNPs)
Surface Plasmon Resonance (SPR):
- When light hits AuNPs, surface electrons oscillate collectively.
- This makes their color different from bulk gold (red, purple, blue).
- This property is highly useful in biosensors, imaging, and diagnostics.
High Surface Area to Volume Ratio:
- Due to their small size, the surface area is very large.
- Molecules can easily attach to the surface.
- Important for drug delivery and catalysis.
Chemical Stability:
- Gold is an inert metal, making AuNPs stable even in reactive environments.
- They do not degrade easily inside biological systems.
Biocompatibility:
- AuNPs are comparatively non-toxic in the human body.
- Hence, they are used in drug delivery, cancer therapy, and vaccines safely.
Optical Properties:
- AuNPs have powerful optical absorption and scattering.
- Their size and shape influence their color and absorption.
- Useful in imaging, photothermal therapy, and biosensors.
Electrical & Catalytic Properties:
- AuNPs have useful electrical conductivity.
- Their small size enhances chemical reactions (catalysis).
Shape & Size Dependent Properties:
- Their optical, electrical, and catalytic behavior changes depending on their shape (spheres, rods, shells, stars).
- This versatility makes them highly valuable.
S. N. Bose National Centre for Basic Sciences (SNBNCBS)
- Established: 1986
- Named after: Famous Indian physicist Satyendra Nath Bose
- It is an autonomous research institute.
- Location: Salt Lake, Kolkata, West Bengal
- Administration: Functions under the Department of Science & Technology (DST), Government of India
- Fields of Work: Conducts advanced research in basic sciences, particularly in physics, chemistry, biology, and interdisciplinary areas.